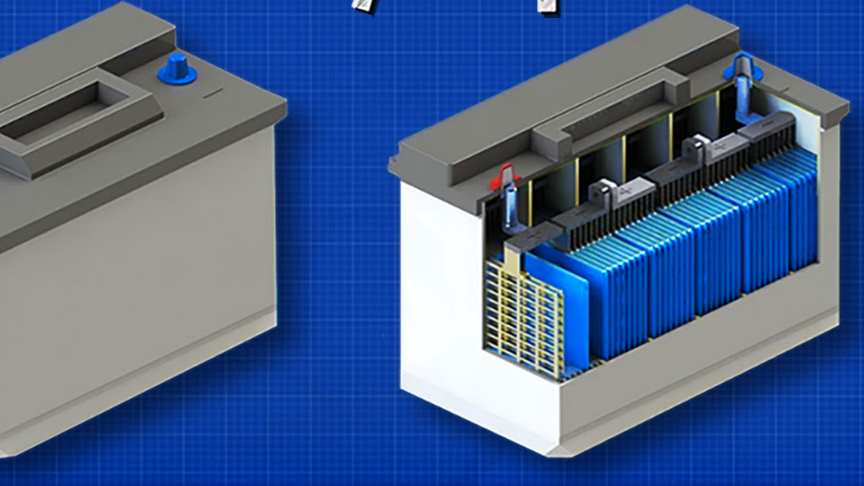
An automobile battery is an example of a wet cell battery, with six cells. Each cell of a lead storage battery consists of alternate plates made of a lead alloy grid filled with sponge lead (cathode plates) or coated with lead dioxide (anode).Each cell is filled with a sulfuric acid solution, which is the electrolyte.
source/image(PrtSc): The Engineering Mindset
Initially, cells each had a filler cap, through which the electrolyte level could be viewed and which allowed water to be added to the cell. The filler cap had a small vent hole which allowed hydrogen gas generated during charging to escape from the cell. Watch the video from The Engineering Mindset for more info:
How does a car battery work, learn from the basics where we use and battery and how batteries work.With thanks to Squarespace for sponsoring this video.The cells are connected by short heavy straps from the positive plates of one cell to the negative plates of the adjacent cell.
Advertisement
A pair of heavy terminals, plated with lead to resist corrosion, are mounted at the top, sometimes the side, of the battery.The result is a chemical reaction that produces electrons. The electrons race around the plates and generate electricity. The electricity flows out of the battery terminals to start your engine./wikipedia