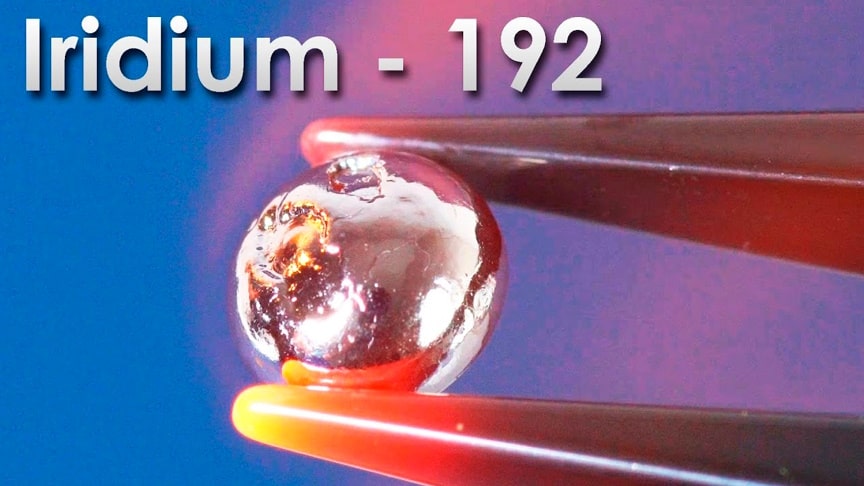
Iridium is a transitional metal, which is located in the middle of the periodic table, below rhodium. If we take a look at the prevalence of all elements in the earth’s crust, Iridium holds the last place, that is a billion atoms of all that there is and only one atom of iridium. This metal is 40 times rarer than gold.In much higher concentrations iridium is found in meteorites and also in the depths of the Earth, in magma.
source/image: Thoisoi2 – Chemical Experiments
Interestingly enough, in the layers of rock sediments, though more precisely in the formation of clay, that is aged about 66 million years there were found high concentrations of iridium and this can indicate the collision of Earth with a huge meteorite in the past, which in theory was the cause of the death of the dinosaurs. In it’s appearance iridium is a shiny metal that does not oxidize in air.
This metal has almost the highest density of all metals, just 0.12% lower than that of osmium – the most dense metal.In this tiny tiny metal droplet, which is of the size of a match head, we have 1 gram of iridium. To help you understand how high is the density of iridium, I will show other metals with the same mass for comparison. Lead, copper, gallium, zinc, magnesium, and the lightest metal – lithium.
Advertisement
The volumes of the first and last metal differ by about 30 times, although their mass is the same. Iridium is also a very hard metal that is firmer than the solid steel in 1.5 (one and a half) times. Iridium, in addition to its rarity is even the most stable metal that does not oxidize in air up to 2000 degrees, and is not soluble in either acid or aqua regia. Iridium can only react with the fluorine at temperatures of about 600 degrees.